Application of 2-Imidazolidone (ethylene urea) in Wet Headliner Process for Formaldehyde Remove
Abstract.
In order to solve the problem of aldehyde in car headliner products, 2-imidazolidone (ethylene urea) was added to the production of headliner samples by wet process. The effect of the addition of ethylene urea on the formaldehyde and acetaldehyde in the samples was tested. The changes of aldehyde in the samples with the addition of 2 imidazolidone (ethylene urea) over time were also investigated and analyzed.
The study showed that by adding 2-imidazolidone (ethylene urea) to the headliner samples, the aldehyde of the products could be significantly reduced. The addition process is easy to operate. After the treatment, the aldehyde of the product can be maintained at a low level for a long time.
Keywords: headliner, 2-imidazolidone (ethylene urea), aldehyde.
1. Introduction.
Automotive interior headliner is an important interior product in the car, while improving the aesthetics of the car, both sound insulation, heat insulation and other functions. Headliner can well enhance the driving comfort of the occupants.
The production process of headliner is mainly divided into “dry” and “wet” two kinds of processes. The “wet” process is gradually becoming one of the main processes for the production of headliners, with its better dimensional stability and lightweight advantages.
However, the “wet” process also has its drawbacks. Because in the production process, thermoplastic semi-rigid polyurethane foam and polyurethane glue are used. Both of them will release most of the aldehydes.
In addition, formaldehyde is often used as a fabric smoothing agent in the production of headliner fabrics from upstream suppliers. Therefore, the aldehydes in the car interior headliner produced by the “wet” process are very likely to exceed the standard.
The aldehydes in the car interior roof exceed the standard, on the one hand, will affect the health of the production operators. On the other hand, the products with excessive aldehydes need to be post-treated by hot-drying, resulting in increased processes and wasted energy.
Therefore, it is a difficult problem to reduce the aldehydes in the actual production of car headliners.
Ethylene urea, also known as 2-imidazolidione. The appearance is needle-like crystals. It has a low odor and is highly soluble in water. 2-imidazolidinone is widely used in aldehyde removal applications. It is a high performance aldehyde scavenger.
Ethylene urea can react with formaldehyde and acetaldehyde at room temperature. 2-imidazolidione can reduce the aldehyde content of products when added to the components of products.
In this research, a “wet” process was chosen. Different concentrations of ethylene urea aqueous solutions were used in the production process to replace the water in the “sprinkling” process. To study the changes of formaldehyde and acetaldehyde content in the finished car headliner. Among them, the simulated “one-step wet” process of the headliner is as follows: semi-rigid polyurethane foam sheet feeding → polyurethane glue rolling → water sprinkling/sprinkling ethylene urea aqueous solution → raw material stacking → transfer material → composite molding (hot mold) → shaping → water cutting → edge wrapping + adhesion parts.
2. Experimental section.
2.1 Main raw materials.
a. Ethylene urea.
b. Deionized water.
c. Glass fiber felt (gram weight 160 g/m2).
d. PP non-woven fabric (gram weight 25 g/m2).
e. Polyurethane semi-rigid foam (density 23 g/cm2, thickness 8 mm).
f. Automotive headliner fabric (gram weight 250 g/m2).
2.2 Main equipment.
a. Flat plate vulcanizing machine (YS30TV).
b. High performance liquid chromatograph (1260).
c. Walk-in sampling chamber (32 m3).
2.3 Preparation of ethylene urea aqueous solution.
At room temperature, put a certain proportion of Ethylene urea and deionized water into a three-neck flask with stirring device. Start to stir and disper for 5 min. Then the different concentrations of vinyl urea solutions were quickly transferred to closed plastic bottles for storage.
2.4 Sample preparation method.
The samples were prepared by simulating the “one-step wet process” for automotive interior headliners as follows.
a. Cut A4 size (210 mm×297 mm) glass fiber felt, polyurethane semi-rigid foam, non-woven fabric and fabric separately and set aside.
b. Spray polyurethane glue on both sides of polyurethane semi-rigid foam, 5 g for both upper and lower sides, total 10 g. After spraying glue, quickly spray EU-0 solution on both sides of foam, 1 g for both upper and lower sides, total 2 g.
c. Stacking of raw materials. Lay the non-woven fabric, fiberglass felt, polyurethane semi-rigid foam, fiberglass felt and fabric in order from bottom to top. And then the stacked raw materials are transferred together into the flat vulcanizer. The mold is pressed under the specified parameters of mold gap temperature, pressure and time. The sample is then removed. Mark the sample as “Sample EU-0”.
d. Repeat the above steps. In step 2, replace EU-0 solution with EU-1, EU-2, etc. to prepare other samples. The samples are also labeled as “Sample EU-1”, “Sample EU-2” …… and “Sample EU-6” in that order. The samples were left at room temperature for 72 h and then tested (Table 1).
| Solution No. | EU, g | Deionized water, g | EU (mass ratio), % |
|---|---|---|---|
| EU-0 | 0 | 500 | 0 |
| EU-1 | 3 | 497 | 0.6 |
| EU-3 | 15 | 485 | 3 |
| EU-4 | 30 | 470 | 6 |
| EU-5 | 50 | 450 | 10 |
| EU-6 | 80 | 420 | 16 |
2.5 Test method.
The prepared samples were tested according to CVTC 54072-2015 “Test method for volatile organic compounds and aldehydes and ketones in automotive parts (bag method)”.
a. Take a 10L “Tedlar” sampling bag. Fill the bag with the “Sample EU-0” prepared in step 1.4. Then close the bag.
b. Use high purity nitrogen to replace the gas in the bag. Fill the bag with about 5L of high purity nitrogen. Then pull out the gases. Repeat the operation 3 times.
c. Accurately fill with 5L of high purity nitrogen and place the sample bag with the sample into the walk-in sampling chamber. Heat at 65°C for 2h. Afterwards, collect 2L of aldehydes with the DNPH tube.
d. The trapped DNPH tube was eluted. The eluate will be processed and tested for aldehyde content by liquid chromatography.
e. Repeat the above steps. Complete the aldehyde content test for other samples.
f. Take blank samples EU-0, EU-4 and store them in a general laboratory environment. Take out after a certain time interval. Test the samples for formaldehyde and acetaldehyde content. Research the change of aldehyde content with time.
3. Results and Discussion.
3.1 The effect of ethylene urea concentration on the formaldehyde and acetaldehyde content of the samples.
Because ethylene urea contains secondary amine groups. The product of reaction between vinyl urea and aldehydes also contains hydroxyl groups. Both can react with the isocyanate group in polyurethane. Therefore, in the actual curing process of polyurethane glue, there are three reactions between the isocyanate group and water, secondary amine and the hydroxyl group in the dehyde product. This paper will not go into the details here. Only the effect of theoretical addition of ethylene urea on aldehydes of the samples is discussed.
The reaction equations of ethylene urea with formaldehyde and acetaldehyde are shown in Fig. 1 and Fig. 2 below.
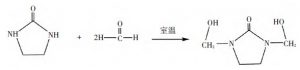
Figure 1, Reaction of ethylene urea with formaldehyde
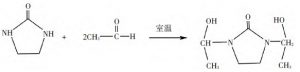
Figure 2, Reaction of ethylene urea with acetaldehyde
In the “one-step wet” process, ethylene urea was added to the samples by replacing the pure water in the sprinkling step with a solution of vinyl urea. The amount of ethylene urea in the samples is shown in Table 2.
| Sample Name | Ethylene urea content, g |
|---|---|
| Sample EU-0 | 0 |
| Sample EU-1 | 0.012 |
| Sample EU-3 | 0.060 |
| Sample EU-4 | 0.12 |
| Sample EU-5 | 0.20 |
| Sample EU-6 | 0.32 |
The formaldehyde and acetaldehyde contents of the samples in Table 2 were tested by the “bag method”. The relationship between the content of formaldehyde and acetaldehyde and the content of ethylene urea is shown in Figure 3.
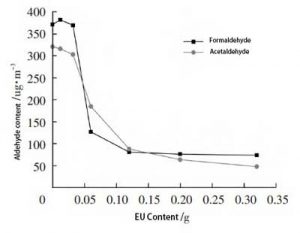
Figure 3, Effect of ethylene urea content on aldehyde content of samples
From Figure 3, it can be seen that the amount of formaldehyde in the samples shows a decreasing trend with the increase of the content of ethyleneurea. This indicates that the reaction of ethylene urea with formaldehyde in the sample occurred. And with the increase of ethyleneurea content, the amount of formaldehyde reacted increases and the amount of formaldehyde remaining in the sample decreases.
The formaldehyde content did not change significantly for the ethylene urea content of 0.032 g. The formaldehyde content of the EU-1 sample was also slightly higher compared to that of the EU-0 sample. This is because although the raw materials and dimensions of the prepared samples are the same. However, the content of aldehydes contained in the samples may still be slightly different. The content of vinyl urea is less and the elimination effect of formaldehyde is not obvious.
The amount of formaldehyde decreases rapidly between 0.032~0.12 g of ethyleneurea content. It indicates that this content of ethylene urea is the best amount for eliminating formaldehyde.
Above the content of 0.12 g of ethyleneurea, the decreasing trend of formaldehyde was significantly slowed down. It means that most of the formaldehyde in the sample has already participated in the reaction, and the concentration of the remaining formaldehyde is too low for the reaction to continue.
As can be seen in Figure 3, the amount of acetaldehyde in the samples decreased with the increase in the content of ethylene urea. The trend of change was similar to that of formaldehyde. It indicates that ethylene urea also reacted with acetaldehyde in the samples. The content of ethylene urea between 0.032 and 0.20 g is the best amount to eliminate acetaldehyde.
In summary, the best dosage range of ethylene urea for the elimination of aldehydes in the samples was between 0.032 and 0.20 g.
3.2 Variation of aldehyde content of samples after ethylene urea treatment with time.
Blank samples EU-0, EU-4, were stored in a general laboratory environment. Take them out after a certain time interval. The formaldehyde and acetaldehyde contents of the samples were tested. The change of aldehyde content with time was studied. The contents of formaldehyde and acetaldehyde are shown in Figure 4 and Figure 5, respectively.
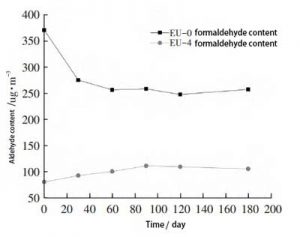
Fig. 4, Variation of aldehyde content substance of samples after ethylene urea treatment with time (formaldehyde)
The EU-4 samples showed an increase in formaldehyde levels between 30 and 90 days, with natural degradation producing aldehydes. This should be the main influencing factor. However, the amount of formaldehyde did not change significantly throughout the testing process.
The formaldehyde content of EU-4 samples was significantly lower than that of EU-0 samples throughout the testing cycle. This indicates that the samples treated with ethylene urea can maintain the low formaldehyde content for a longer period of time.
As can be seen in Figure 5, the formaldehyde content of EU-0 samples decreased slightly during the 60-day test period. After that, it showed a more moderate change from high to low. The reason for the change in formaldehyde is similar to that described earlier. The difference is that the boiling point of acetaldehyde is higher than that of formaldehyde, and its volatility is relatively weak. Therefore, it did not show a significant downward trend in the early tests.
The EU-4 samples showed an increase in acetaldehyde content from 30 to 60 days. The natural degradation of acetaldehyde should be the main influencing factor. The amount of acetaldehyde also did not change significantly throughout the test. The acetaldehyde content of the EU-4 samples was significantly lower than that of the EU-0 samples throughout the test period. This indicates that the ethylene urea-treated samples can maintain a low acetaldehyde content for a longer period of time.
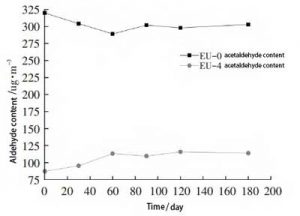
Figure 5, Variation of aldehyde content substance of samples after ethylene urea treatment with time (acetaldehyde)
4. Conclusion.
a. 2-imidazolidone (ethylene urea) has low odor and is very soluble in water. In this study, 2-imidazolidone was firstly formulated into different concentrations of aqueous solutions. After that, the raw materials commonly used in automotive interior headliners were used. The “one-step wet process” for automotive interior headliners was simulated. By replacing pure water with an aqueous solution of ethylene urea in the water sprinkling step. Samples of automotive interior headliner composites with different vinyl urea contents were prepared.
b. The formaldehyde and acetaldehyde contents of the samples with different 2-imidazolidone contents were tested by the “bag method”. The test results showed that the content of formaldehyde and acetaldehyde in the samples could be effectively reduced by vinyl urea. As the amount of 2-imidazolidone increased, the formaldehyde and acetaldehyde content of the samples decreased. The optimum dosage is about 0.12~0.2 g for A4 size samples.
c. The changes of formaldehyde and acetaldehyde contents in the blank samples and the best samples were tested with time by the “bag method”. The results showed that the changes of formaldehyde and acetaldehyde contents of the 2-imidazolidone treated samples were not significant with time. The samples remained persistently low in aldehyde content under general laboratory storage environment.
In summary, it can be seen that 2-imidazolidone can effectively reduce the aldehyde content of headliners when used in the “one-step wet” process for automotive interiors. The use of 2-imidazolidone can improve the air quality in the car and is an effective way to control the aldehyde content of the product.



